Project
Aisa Bottled Water Factory
December 15th, 2024 Asia
- Industry:
Bottled Water Plant
- Location:
Asia
- Cleanroom:
ISO Class 7
- Size:
1,170m²
- Duration:
6 Months
- Key features:
Project Details
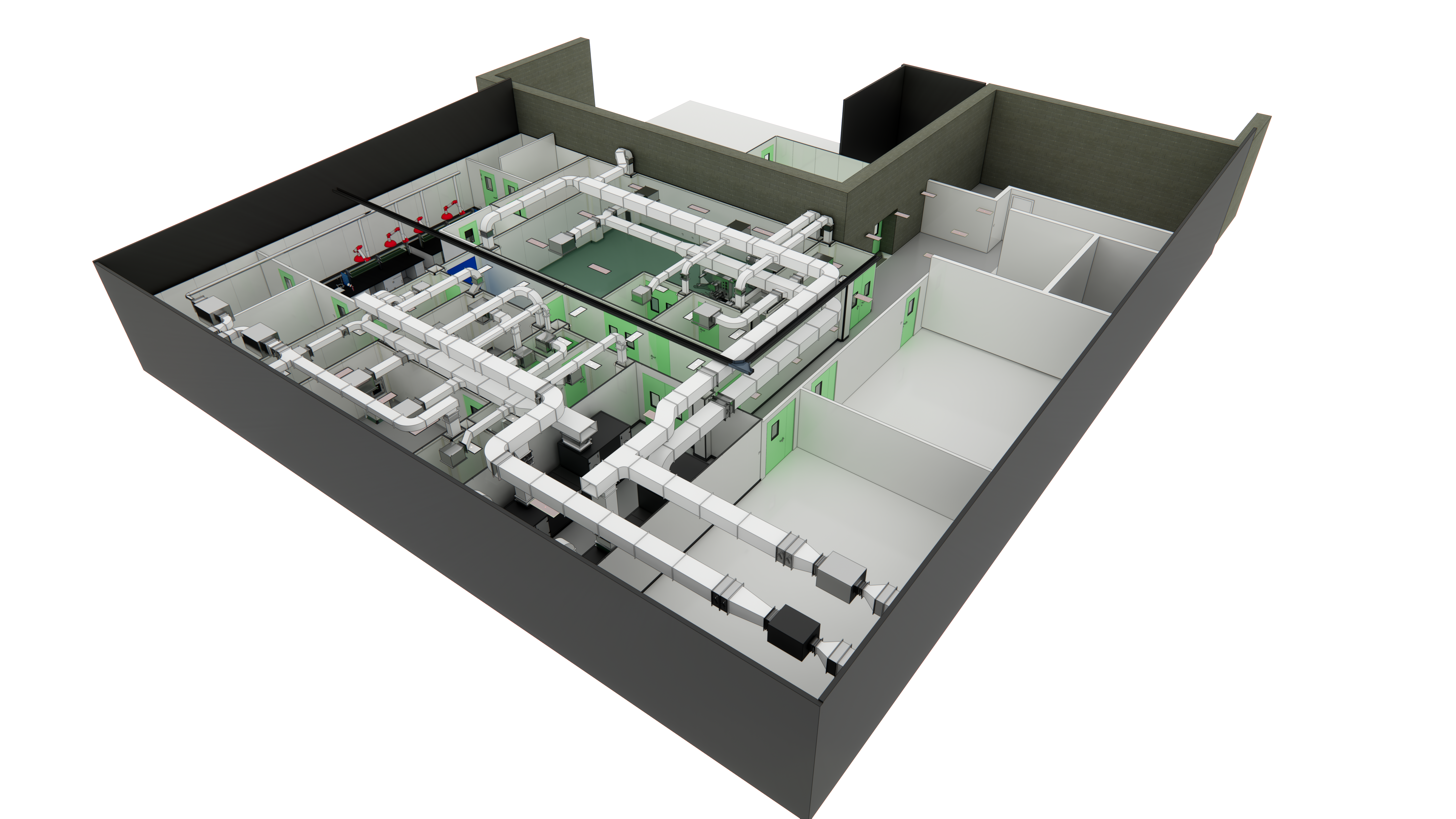
Our team was commissioned by a leading Asian bottled‑water producer to deliver a fully turnkey, two‑story ISO 7 cleanroom complex—bringing together process, quality‑assurance, and R&D functions under one roof. From sugar‑handling to microbiological testing and even a dedicated positive‑control suite, we engineered every element to safeguard product purity while streamlining installation and startup.
Project Video
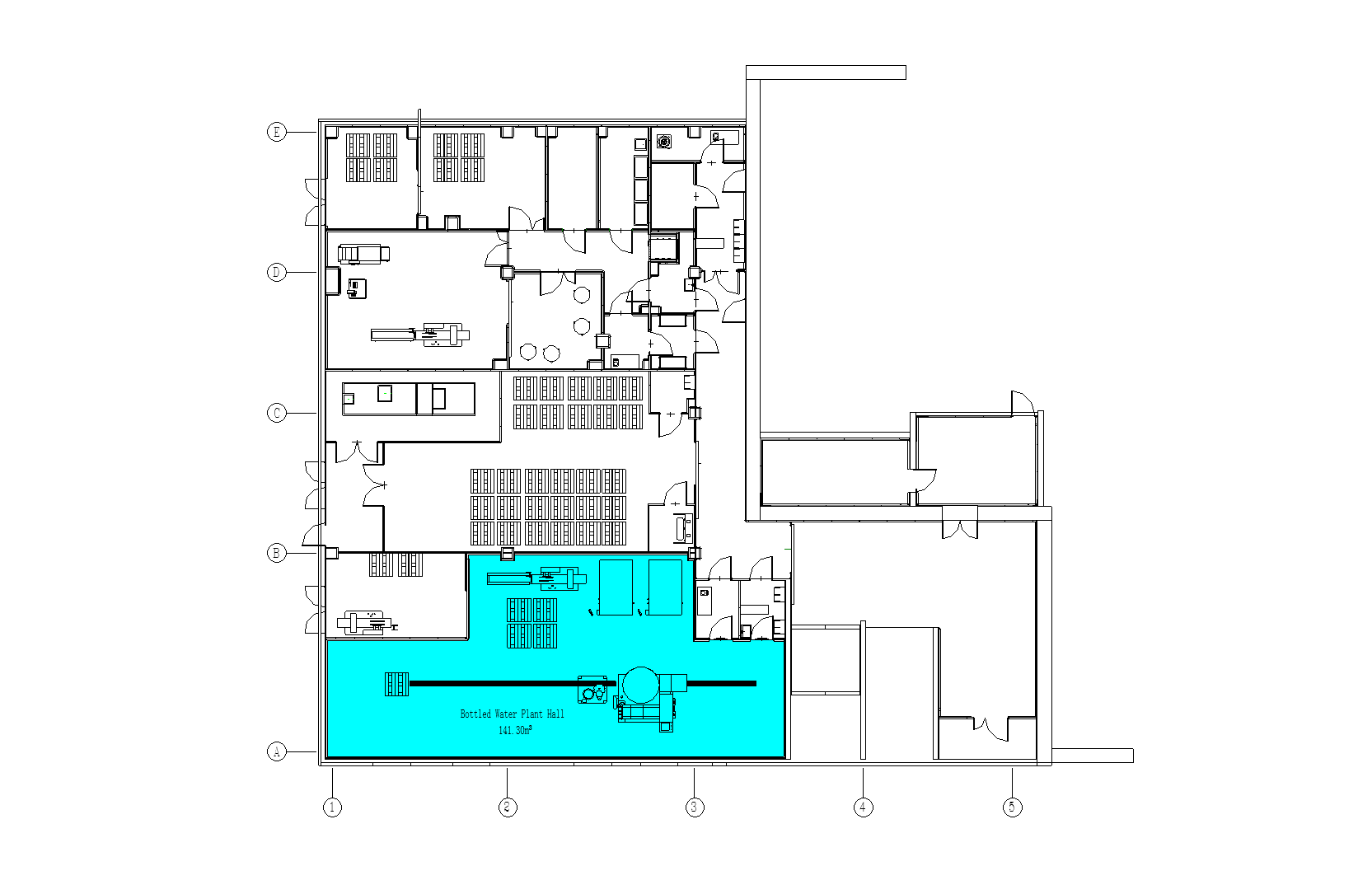
Bottled Water Plant Hall
The Bottled Water Plant Hall is the central production zone of a bottled water facility, integrating water treatment, filling, packaging, and quality control processes.
- 1.Functional Zoning: –Water Purification Area: Equipped with reverse osmosis (RO) systems, sand filters, and UV sterilization to ensure water purity . –Filling & Packaging Zone: Automated filling machines (e.g., rotary fillers) and capping units handle bottle filling and sealing, achieving capacities up to 15,000 bottles/hour . –Cleanroom Environment: Maintains ISO Class 8 cleanliness (≤3.5×10⁶ particles/m³) with HEPA-filtered air and controlled pressure differentials to prevent contamination .
- 2.Process Flow: –Raw water undergoes pre-filtration → activated carbon treatment → RO purification before entering sterile storage tanks . –Bottles are cleaned (via high-pressure rinsing), filled, capped, labeled, and packed in a linear workflow to minimize cross-contamination .
- 3.Key Equipment: –Bottle Blowing Machines: Produce PET bottles on-site to reduce logistics costs . –Air Showers & Pass-Through Systems: Installed at entry points to remove dust from personnel and materials .
- 4.Logistics & Safety: –Material Flow: Raw materials (e.g., bottle preforms, caps) and finished products are segregated in designated storage areas . –GMP Compliance: Flooring uses anti-static, corrosion-resistant materials, and drainage systems prevent water pooling .
- This hall serves as the operational backbone, combining advanced automation with strict hygiene protocols.
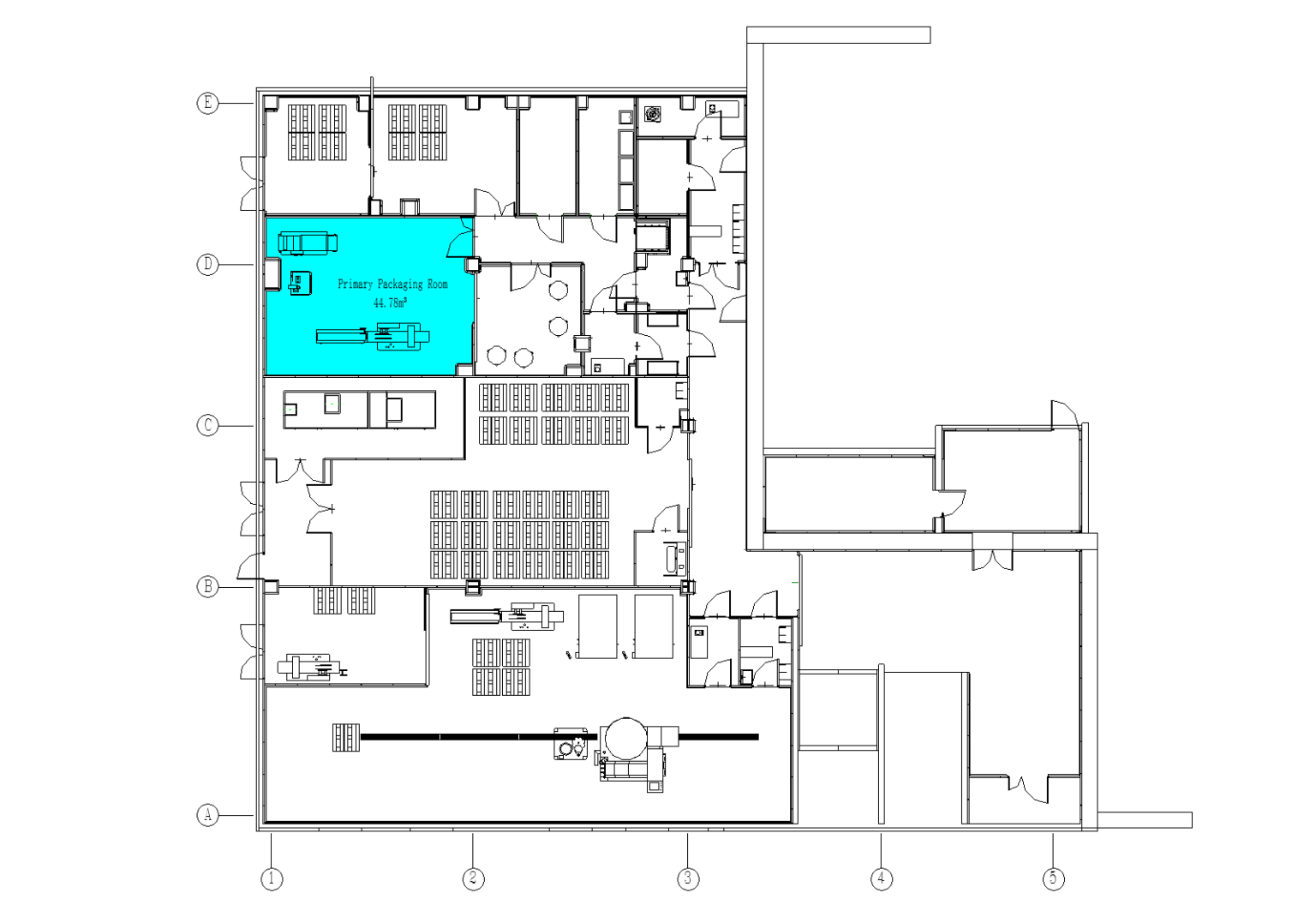
Primary Packaging Room
A primary packaging room is a designated area in manufacturing facilities where products are directly enclosed in their first layer of packaging (e.g., bottles, blister packs, or pouches). This space focuses on ensuring product integrity, hygiene, and compliance with safety standards. Key features include:
- 1.Direct Contact with Products: Primary packaging materials (e.g., vials, cans, or sachets) directly touch the product to preserve freshness, prevent contamination, and maintain quality .
- 2.Core Operations: Activities like filling, sealing, labeling, and initial quality checks are performed here. For example, pharmaceutical tablets are sealed in blister packs, and beverages are bottled and capped .
- 3.Sanitation Standards: Often requires controlled environments (e.g., ISO Class 8 cleanliness for pharmaceuticals or food) to meet regulatory requirements .
- 4.Equipment: Utilizes machines such as filling machines, cappers, and induction sealers, tailored to handle specific materials like glass, plastic, or foil .
- 5.Industry Applications: Critical in food, pharmaceuticals, cosmetics, and chemicals, where packaging directly impacts product safety and shelf life .
- This room serves as the first protective barrier before secondary packaging (e.g., boxing or palletizing) .
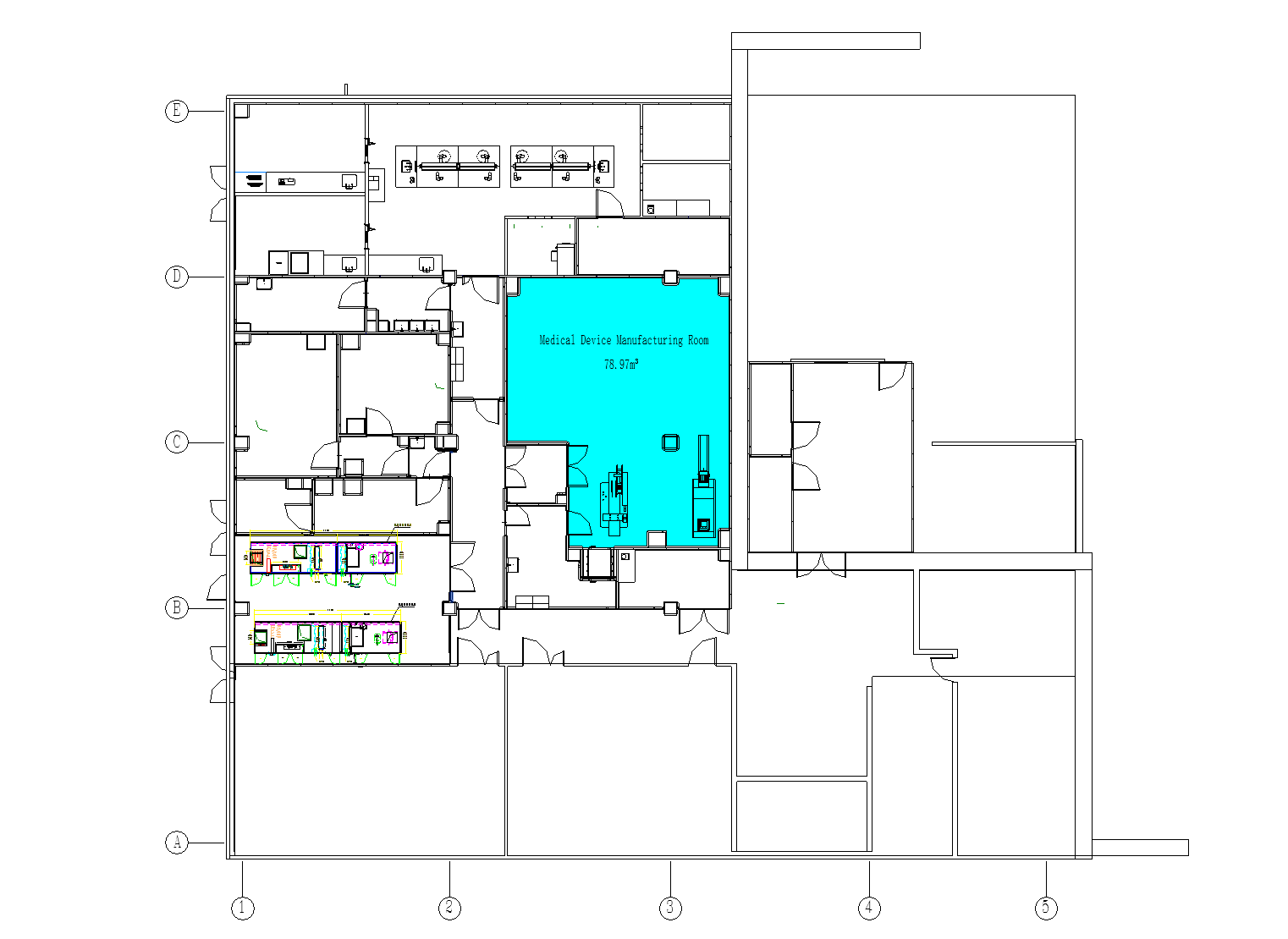
Medical Device Manufacturing Room
A medical device manufacturing facility is a specialized production environment designed to ensure the safety, precision, and regulatory compliance of medical devices, ranging from surgical instruments to implantable products like pacemakers and orthopedic implants.
- 1.Core Areas: –Assembly Area: Manual or automated assembly of device components, often under controlled atmospheres . –Sterilization Room: Equipped with ethylene oxide (EO) sterilizers or gamma radiation systems, validated for load configurations and microbial kill rates . –Packaging Zone: Sealing and labeling in ISO Class 7/8 environments to ensure sterility until use .
- 2.This facility integrates advanced engineering, rigorous protocols, and continuous monitoring to meet global standards like CE Marking and MDR/IVDR .
Our process
How to Start Your Project with Yaguan?
Follow the steps and get the satisfactory result in the shortest path
- Understand your requirements
- Develop the design
- Manufacturing and verification
- Delivery and installation guidance