Project
Medical Device Manufacturer Production Facility Cleanroom
- Industry:
- Location:
- Cleanroom:
- Size:
- Duration:
- Key features:
Project Details
By combining industry leading cleanroom engineering with cutting edge BIM workflows and modular construction, we enabled our customer to establish a world class injection molding facility that is not only compliant today but also fully adaptable for tomorrow’s growth.
Material Supply & Turn‑Key Delivery
- All cleanroom consumables—antimicrobial wall panels, coved vinyl flooring, stainless‑steel radii, high‑efficiency lights—were specified, procured, and delivered on a just‑in‑time schedule.
- Our field engineers oversaw panel installation, ductwork hookup, pressure testing, particle‑count certification, and final commissioning in under 12 weeks.
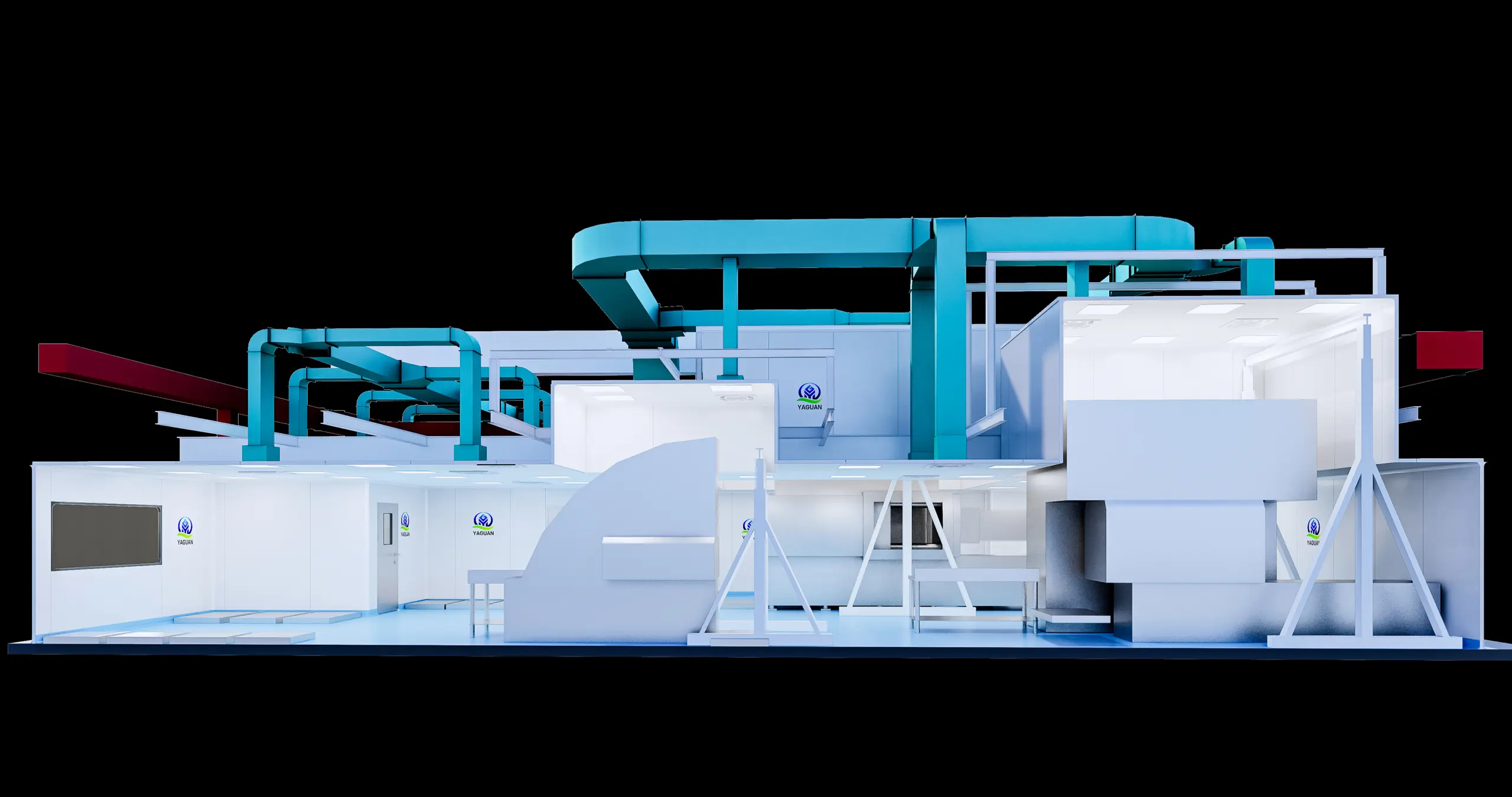
Production Workshop
Key Project Highlights
BIM‑Based Visualization & Coordination
3D model parameterization allowed real‑time clash detection across structural, MEP and architectural trades, reducing RFIs by 60%.
Live model views let the customer “walk through” the production flow, verify ergonomics around press machines, and pre‑approve finishes before procurement.
Modular Installation Strategy
Pre‑fabricated wall and ceiling panels with integrated door frames and glazing reduced on‑site labor by 40%.
“Plug‑and‑play” HEPA‑filter modules were factory tested for airflow, pressure drop, and leak integrity—simply bolted into place and connected to ductwork.
HVAC System
Key Project Highlights
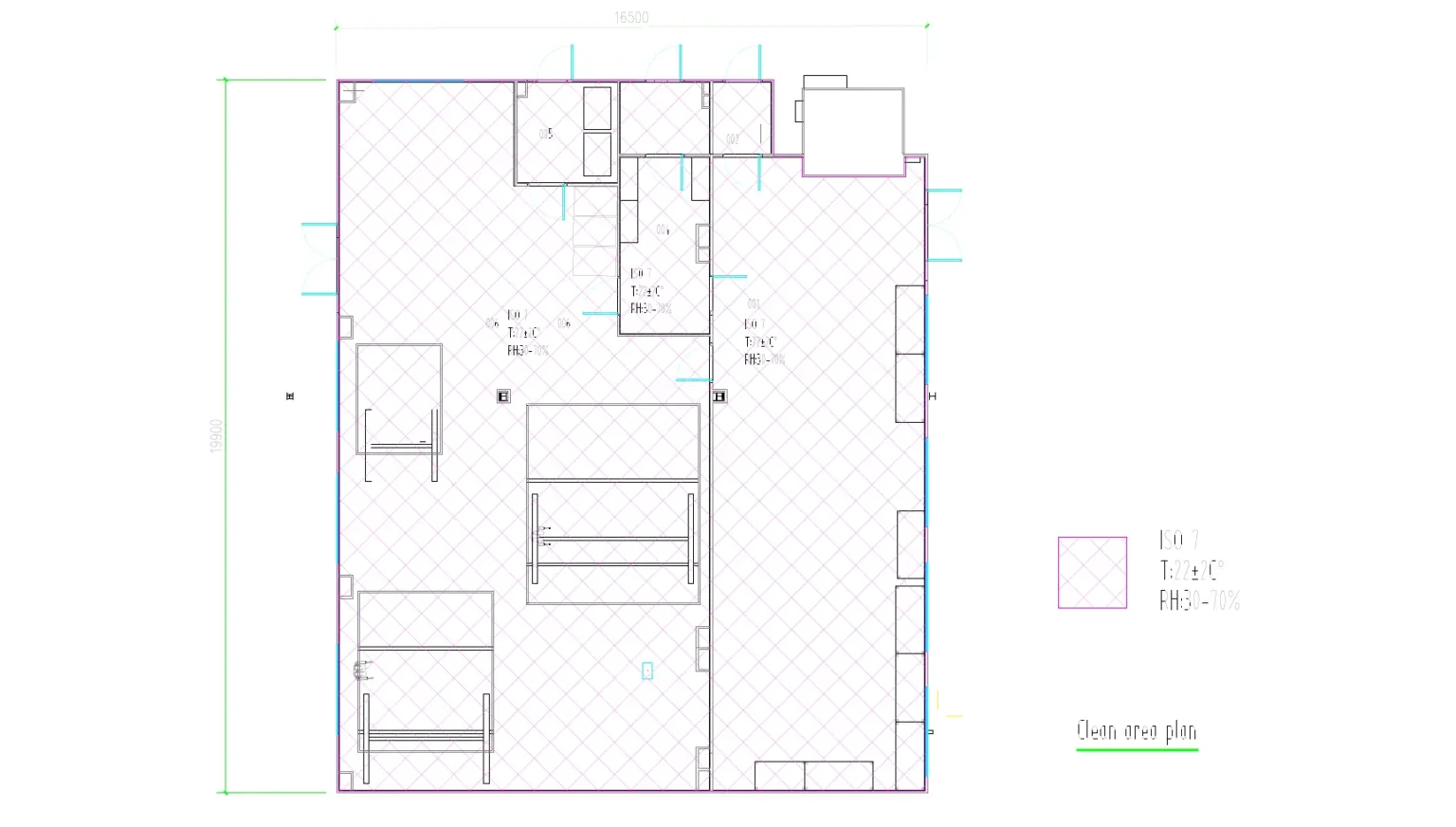
ISO 7 Cleanroom Design
- Maintains ≤ 352,000 particles ≥ 0.5 µm per m³ to meet both EU GMP Annex 1 and ISO 14644‑1 standards.
- Zone layout includes dedicated gowning, airlock, and material pass‑throughs to prevent cross‐contamination.
HVAC & Process Air System
- Dual‑stage filtration (pre‑filter + H14 HEPA) achieves ISO 7 air cleanliness while maintaining ± 5 Pa differential pressure to adjacent non‑classified areas.
- Redundant AHUs with variable‑frequency drives provide precise temperature (22 ± 2 °C) and relative humidity (50 ± 5 %) control, critical for consistent molding cycle times and part quality.
Business Benefits for the Customer
- Speed to Market: Rapid, module‑based installation cuts build‑out time, enabling pilot molding runs just three months after ground‑break.
- Regulatory Confidence: Delivery of a validated cleanroom that easily passes both EU GMP Annex 1 audits and upcoming MDR requirements.
- Scalability: The BIM library of parametric cleanroom modules can be re‑deployed or re‑sized for future expansions—whether to accommodate additional presses or to add a Class 8 secondary packaging zone.
- Total Cost Control: Factory‑certified modules minimize on‑site labor risks and rework, reducing overall installation cost by an estimated 15 %.
How to Start Your Project with Yaguan?
- Understand your requirements
- Develop the design
- Manufacturing and verification
- Delivery and installation guidance